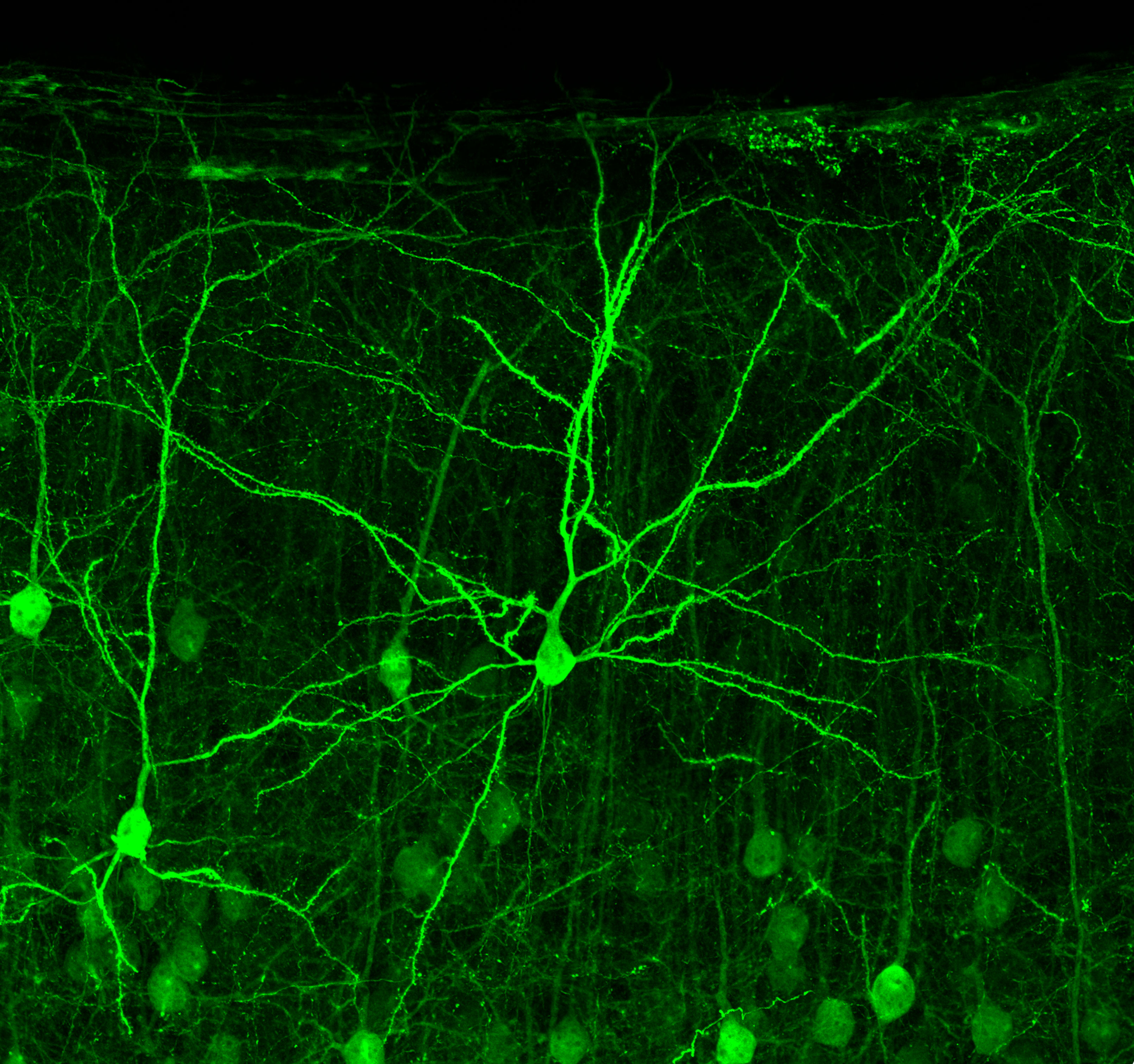
Exploring Cell-Cell Communication in Brain Development
Our lab is focused on uncovering how precise cellular interactions shape the developing brain—and how disruptions in these processes can lead to neurological disorders. In the cerebral cortex, billions of cells must coordinate to form functional circuits. Cell adhesion not only drives synapse formation but also influences neurogenesis, neuronal migration, and dendritic growth.
We study adhesion proteins of the cadherin superfamily, with a particular focus on delta-protocadherins (δ-Pcdhs), which are strongly expressed in the developing and adult brain. These proteins have been linked to key developmental events and are implicated in various brain disorders, yet much remains unknown about their specific roles.
A central focus of our work is PCDH19, an X-linked δ2-protocadherin whose mosaic mutation causes a rare form of childhood epilepsy. By combining genetics, molecular biology, and in vivo approaches, we aim to understand how δ-Pcdhs contribute to circuit formation and brain function.
01.
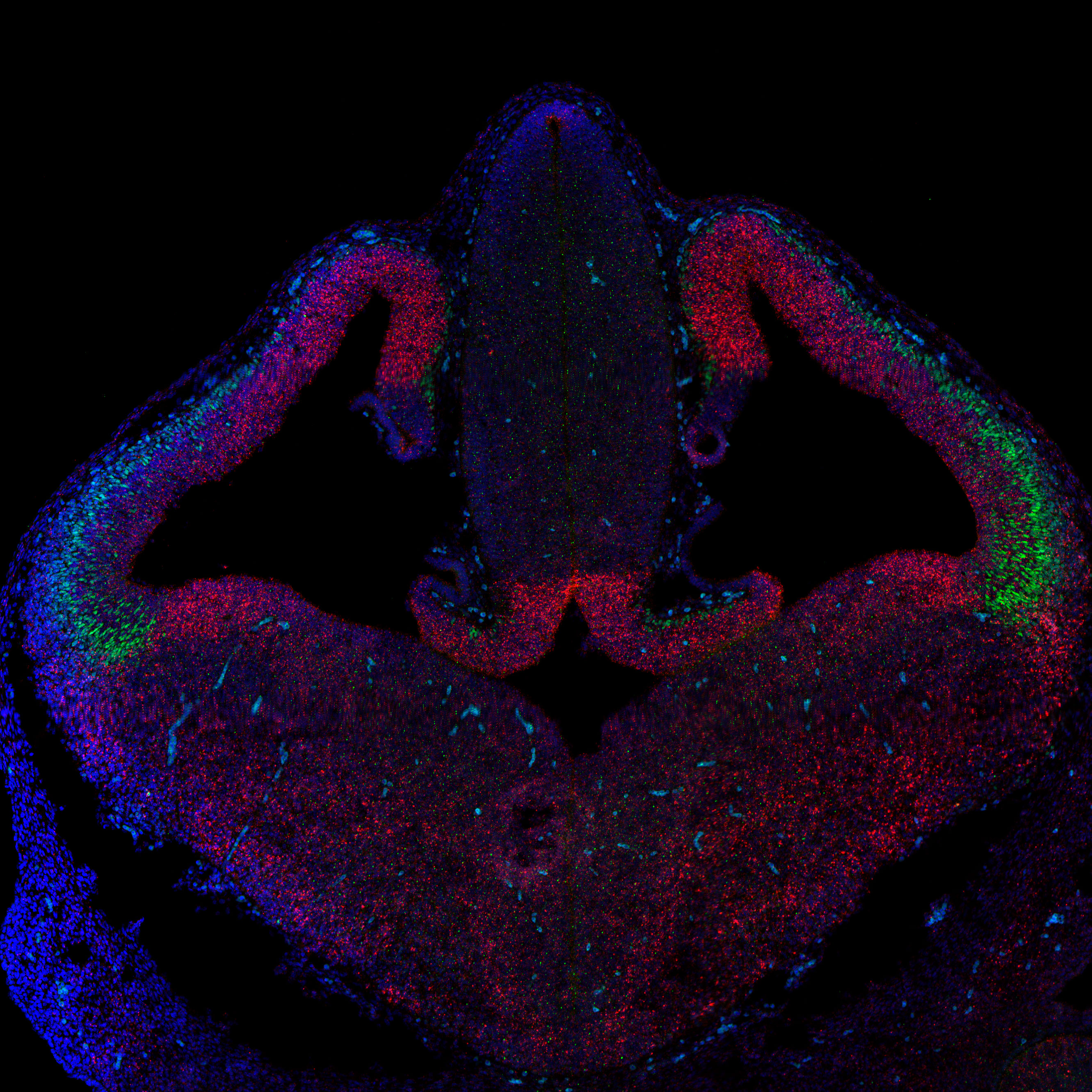
Cell adhesion in the regulation of synaptic function: the role of PCDH19
Mutations in Protocadherin 19 (PCDH19) cause DEE9, a rare epilepsy syndrome that mainly affects girls in early infancy. While seizures often improve with age, cognitive and psychiatric symptoms can persist. The disease uniquely affects females and mosaic males, due to a phenomenon called cellular interference—where mixed populations of normal and mutant cells disrupt brain function.
Our lab investigates how PCDH19 contributes to synaptic function and how its disruption leads to neurological symptoms. We focus on four key questions:
1. How does PCDH19 influence calcium signaling and communication between neurons?
2. How does PCDH19 affect the protein composition of synapses?
3. How does the intracellular domain of PCDH19 control dendritic spine density and gene expression?
4. How does PCDH19 loss alter network activity in the brain?
Our work combines molecular biology, proteomics, transcriptomics, and electrophysiology to uncover how PCDH19 shapes neuronal communication and contributes to epilepsy.
02.
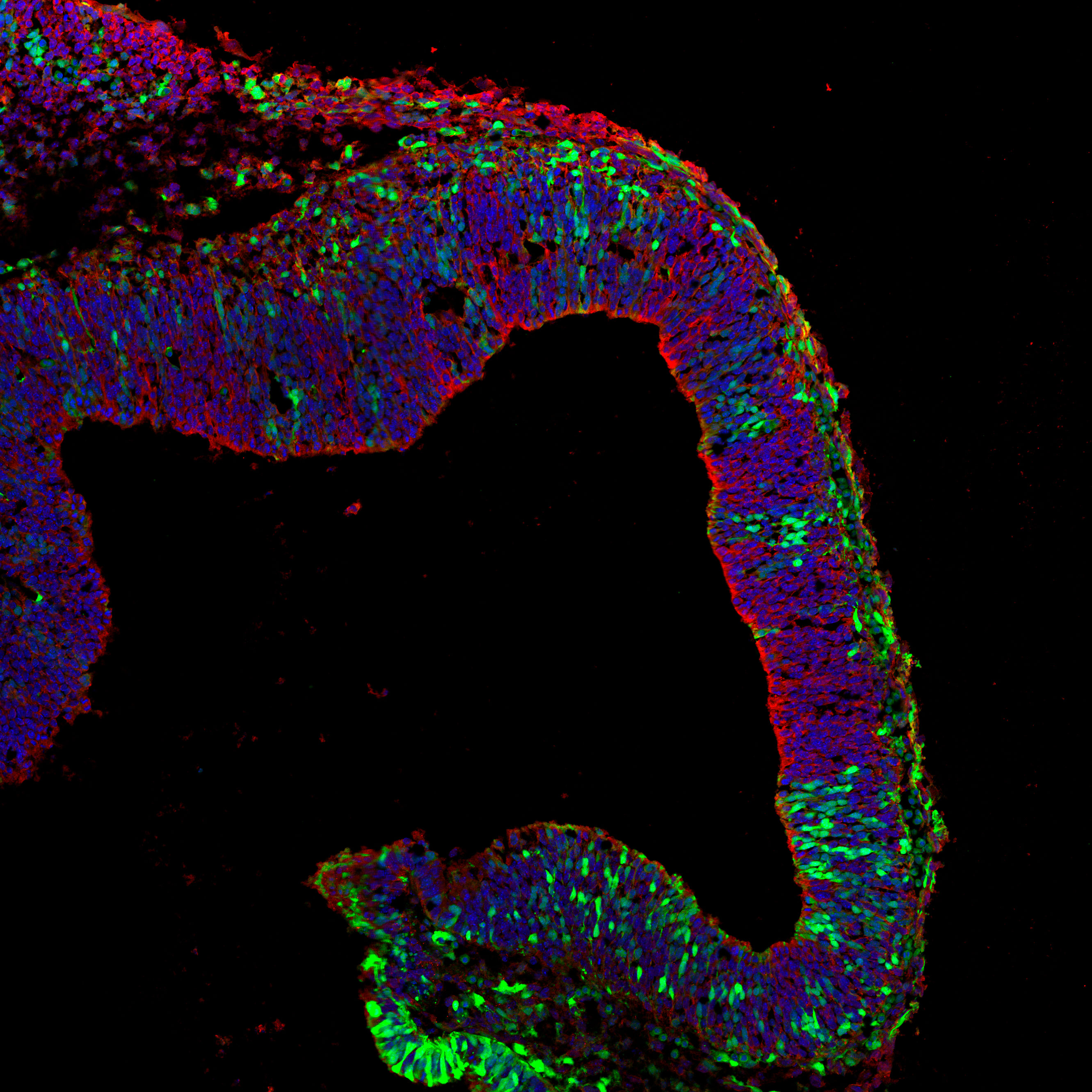
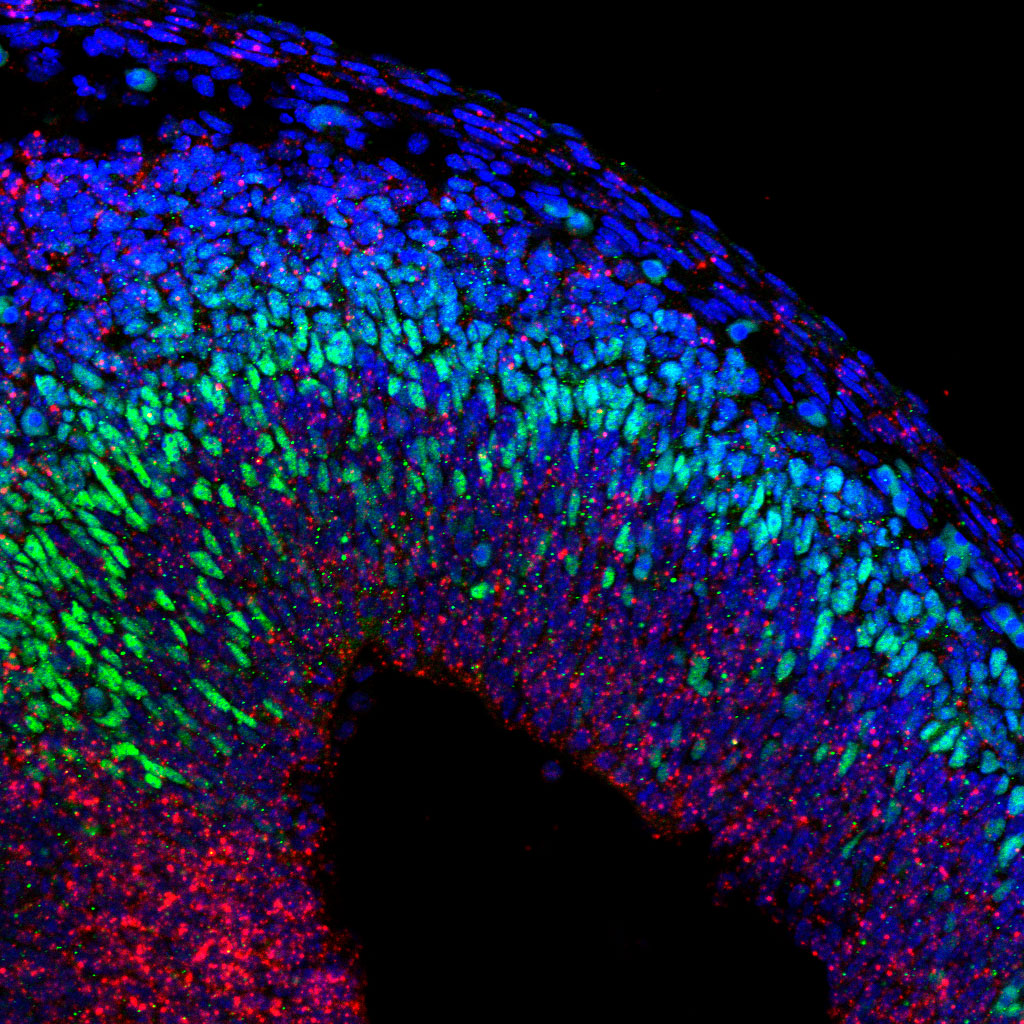
Cell-Cell Communication in Cortical Neurogenesis
The cerebral cortex is essential for higher brain functions such as memory, attention, perception, and language. Its complex structure arises during embryonic development from the tightly regulated expansion and differentiation of neural progenitor cells.
Our lab investigates the mechanisms that control the balance between progenitor proliferation and the generation of diverse neuronal subtypes—key steps in building functional cortical circuits. A central question in developmental neuroscience is how progenitors decide when to switch from self-renewing divisions to neurogenic ones.
Our research has uncovered a novel role for the cell adhesion molecule PCDH19 in this process. We found that neural progenitors alter their neurogenic behavior depending on the presence or absence of PCDH19 in their environment, pointing to a unique form of intercellular communication. We are now working to understand how PCDH19 integrates local signals to regulate progenitor fate decisions and ensure proper cortical development.